Cannabis: a multifaceted plant with endless potentials
Eric Fordjour 1,2*, Charles F. Manful 1, Albert A. Sey1, Rabia Javed1, Thu Huong Pham1, Raymond Thomas 2 and Mumtaz Cheema1*
Cannabis sativa, also known as «hemp» or «weed,» is a versatile plant with various uses in medicine, agriculture, food, and cosmetics. This review attempts to evaluate the available literature on the ecology, chemical composition, phytochemistry, pharmacology, traditional uses, industrial uses, and toxicology of Cannabis sativa. So far, 566 chemical compounds have been isolated from Cannabis, including 125 cannabinoids and 198 non-cannabinoids. The psychoactive and physiologically active part of the plant is a cannabinoid, mostly found in the flowers, but also present in smaller amounts in the leaves, stems, and seeds. Of all phytochemicals, terpenes form the largest composition in the plant. Pharmacological evidence reveals that the plants contain cannabinoids which exhibit potential as antioxidants, antibacterial agents, anticancer agents, and anti-inflammatory agents. Furthermore, the compounds in the plants have reported applications in the food and cosmetic industries. Significantly. Cannabis cultivation has a minimal negative impact on the environment in terms of cultivation. Most of the studies focused on the chemical make-up, phytochemistry, and pharmacological effects, but not much is known about the toxic effects. Overall, the Cannabis plant has enormous potential for biological and industrial uses, as well as traditional and other medicinal uses. However, further research is necessary to fully understand and explore the uses and beneficial properties of Cannabis sativa.
1- Introduction
Throughout human civilization, there has been a pursuit of plants for their unique potential, including medicinal use. Evidence of this dates back to 60,000 years, with a recent discovery of a 5,000-year-old Sumerian clay tablet that confirms the use of medicinal plants in drug production (Sumner, 2000). Natural resources like medicinal plants, also known as green medicine, are gaining popularity worldwide due to their safety, effectiveness, cultural acceptance, and lower risk of adverse effects compared to synthetic medications (Mustafa et al., 2017). Today, traditional botanical medicines are widely used to treat human health problems, with over 80% of the global population depending on them (Mander and Liu, 2010). Cannabis sativa L. (2n ¼ 20) is a well-known plant that has been around since the beginning of time (Small, 2017). This annual plant is a member of the family Cannabaceae and a widespread plant found in varied environments (Andre et al., 2016). It has been used by humans for over 5,000 years and is one of the oldest plant sources of food and fiber (Appendino et al., 2008). The botanical types of Cannabis sativa differ in terms of their chemical content, plant growth habits, agronomic requirements, and processing (Datwyler and Weiblen, 2006). Cannabis flowers and leaves have a distinctive aroma, and the plant’s extracts include a variety of beneficial flavonoids, terpenes, and other compounds that are efficient insecticides, fungicides, and therapeutic agents (Pellati et al., 2018).
The flower, leaves, oil, and trichome of the plant have been shown to be cytotoxic, antimicrobial, antioxidant, antihypertensive, antipyretic, and appetite-stimulating (Russo and Marcu, 2017). The flower extracts with antioxidant activity have been shown to have health-promoting and anti-aging properties, and are utilized to treat a variety of metabolic and chronic disorders, including glaucoma, pain, depression, cancer, liver disease, cardiovascular diseases, inflammation, and metabolic syndrome (Nallathambi et al., 2017). As an agricultural crop, industrial Cannabis (hemp), is a plant that may be harvested for its fiber (Johnson, 2014). While in the cosmetic industry, it is used for skincare products such as anti-aging creams and hair food (Schettino et al., 2021). Traditionally, the seeds are used for making oil, while the leaves were the second most consumed part of the plant and were used in various ways, such as seasoning, baking, flour, and added to meals (Iftikhar et al., 2021; Kuppuram, 2022; Xu et al., 2022).
Even though Cannabis is used in many ways, the drug’s unclear legal status worldwide has made it hard to study for the last century (Smith et al., 2014). In addition, there has not been much information about comprehensive analysis of the plant that can show the plant’s usefulness in all aspects. In this review, Cannabis’ potential is discussed in length to provide thorough and up-to-date information on the Cannabis plants.
2 Ethnobotany of Cannabis
2.1 Ecology and distribution
Cannabis sativa’s origin is unknown, but it is believed to have come from temperate regions in Asia, specifically the southern Caspian region, Siberia, China, or the Himalayas (Minelli, 2015). However, due to widespread transportation and modification by humans over the past 6,000 years, it is challenging to determine its original geographic range or whether a plant collected in nature is a primitive wild type or has been influenced by human domestication (Sharma, 1979). “Weed” is the most common informal name for the marijuana form of Cannabis sativa, and it accurately describes the species as a weed that grows primarily in habitats created or modified by humans (Small, 2015). It can be found in various places such as fields, trash heaps, vacant lots, pastures, ditches, creeks, and open woods. However, it is poorly adapted to infiltrating established perennial stands and typically invades only after the soil has been recently disturbed or plowed (Small, 2017).
Except in drainage channels, where it is extremely well suited, weedy Cannabis sativa is a slow colonizer, spreading slowly throughout the landscape. It is possible to judge the ecology of Cannabis sativa prior to human intervention based on the circumstances and adaptations of existing wild-growing populations of this plant species (Small, 2017). By examining the circumstances and adaptations of these populations, researchers can gain insight into the plant’s natural habitat, growth patterns, and environmental interactions. For example, studying the genetic diversity of wild-growing Cannabis sativa populations can provide information on the plant’s evolutionary history and geographic distribution (Small, 2015). Additionally, analyzing the physical characteristics of wild Cannabis sativa plants, such as their size, leaf shape, and stem structure, can provide clues about their adaptation to various environmental conditions (Ren et al., 2021).
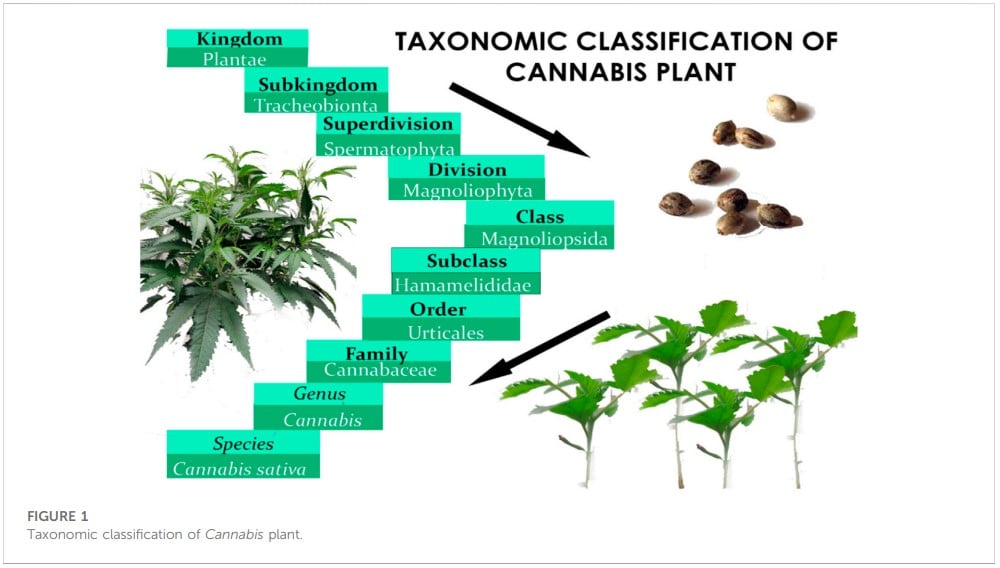
2.2 Taxonomic classification and common names
Before Linnaeus published Species Plantarum in the 18th century, domestic hemp was known by various names, including Cannabis angustifolia, Cannabis sativa, and Cannabis indica (Linnaeus, 1753). Later, Jean-Baptiste Lamarck proposed a division between extensively cultivated Cannabis species in western continents and the wild variety found in India (Erkelens and Hazekamp, 2014). After 50 years, Lindley reclassified Cannabis under Linnaeus’ classification system, affirming the plant’s monospecific status for the rest of the century (Lindley, 2011). Below is the botanical classification of Cannabis plant (Figure 1) (Small, 2017). In the early 20th century, a new species called Cannabis ruderalis emerged, but it was not until 1975 that the restoration of the Cannabis indica species to its current name was proposed (Holland, 2010). Figure 2 presents the name of Cannabis in some popular languages.
Cannabis is a polymorphic plant, and chemotaxonomic markers are effective in differentiating between different Cannabis germplasms and screening for hybrids (Piomelli and Russo, 2016). Small and Cronquist (1976) used biphasic techniques (use of distinct approaches) to identify the four subspecies of Cannabis sativa, including sativa var. sativa, sativa var. spontanea, indica var. indica, and indica var. kafiristanica based on morphological and chemical characteristics such as fruit morphology and THC content (Schultes et al., 1975; Pollio, 2016). Both variants of the subspecies sativa are widely cultivated in North America, Europe, and Asia, and have low intoxicating potential when compared to other Cannabis cultivars (Small and Cronquist, 1976). Meanwhile, the subspecies Indica’s variants have a strong intoxicating potential and are primarily found in the Asiatic Continent (Pollio, 2016; Small, 2017).
2.3 Legality-based classification
Despite being an arbitrary term that does not reflect the drug’s properties, Cannabis is classified as a “narcotic” (i.e., illegal drug) in the legal world (Small, 2017). An illegal drug is defined as a chemical or preparation associated with severe punishments due to its actual or suspected detrimental properties (Smith et al., 2014). Cannabis has been criminalized since the Second World War due to its popular use as a recreational substance, leading to limited research and commercial development in the sector. As a result, research and commercial development on the plant was prohibited for most of the 20th century (Appendino et al., 2014). Cannabis sativa became the most commonly cultivated black market crop in the Western world after World War II, leading to the allocation of significant law enforcement resources to remove the plants (Chouvy,2019). Scientific investigations in Western countries were mostly approved for criminal justice-related forensic studies to assist law enforcement or medical and social-related studies to document and alleviate negative consequences (Chandra et al., 2008).
2019). Scientific investigations in Western countries were mostly approved for criminal justice-related forensic studies to assist law enforcement or medical and social-related studies to document and alleviate negative consequences (Chandra et al., 2008).
Criminalizing Cannabis has led to high law enforcement costs
and social instability, and many jurisdictions are looking to reduce penalties for its possession and consumption (Small, 2015). The legalization of medical Cannabis is widely accepted, but recreational use is still under debate (Cruz et al., 2016). While punishments for illegal drug use have softened in several countries due to increased public acceptance, although, capital punishment is still a possibility in some Asian countries (Chandra et al., 2008; Small, 2017). The decriminalization of Cannabis use is not unique to the North American continent. More than forty countries have legalized the use of marijuana for medical or recreational purposes. Among these countries are Argentina, Germany, Chile, Colombia, and South Africa (Chouvy, 2019). Additionally, Canada, 18 United States states, and two territories—the District of Columbia and the Australian Capital Territory—have legalized Cannabis. New strains are approved for use in Canada until 2023, and Health Canada has issued regulations amending the Cannabis Act and Cannabis Regulations to ensure proper regulation of Cannabis (Canada, 2018; Caplan, 2018).
2.4 Therapeutic based classification
The cannabinoids in Cannabis are unique terpene phenolic substances. Approximately 100 cannabinoids are produced in epidermal trichomes but in small quantities (Mölleken and Theimer, 1997). As discussed by Small (2017), Cannabis’ psychological effects have been ambiguously called “narcotic” in popular, legal, and scientific contexts. Cannabis and opioids are legally grouped, but they are pharmacologically distinct. “Narcotic” comes from “narcosis,” a substance that induces sleep, but it is used to refer to any medicine that induces sleep, stupor, or insensibility (Macdonald and Rotermann, 2017). In moderate amounts, psychoactive cannabinoids such as THC and CBD in Cannabis can induce sedation (Piomelli and Russo, 2016). CBD has a stimulant effect in low and moderate concentrations, and only in high concentrations has a soothing effect (Piomelli and Russo, 2016). Cannabis sativa’s abundant myrcene is likewise sedative (Russo, 2014). There is still some disagreement on how Cannabis should be pharmacologically classified (Kalant, 2010). In some cases, Cannabis has been classified as a sedative-hypnotic-general anesthetic, a mixed stimulant-depressant, a mild hallucinogen, and a psychedelic (Degenhardt et al., 2015). In surgical and dental procedures, it is referred to as a sedative-hypnotic general anesthetic. Cannabis’s psychedelic, hallucinogenic, psychotomimetic, and psychotic properties are misrepresented by terms like “psychedelic” (Brewster, 2019). While “hallucinogenic” is no longer acceptable, “psychoactive,” “euphoric,” or “intoxicating” are the best pharmacological names for Cannabis (Small, 2017; Brewster, 2019). According to Troutt and DiDonato (2015), medical Cannabis users in the United States are characterized by daily dosing and weekly consumption of 6–9g(Ko et al., 2016). In Canada, 42% of medical marijuana patients consume 2 to 3 times a day, and 40% consume more than 14 g per week. In Canada and the United States, most patients inhale (Ilgen et al., 2013; Bonn-Miller et al., 2014).
Surprisingly, only 53% of adult Cannabis users in the United States use Cannabis purely for recreational purposes, while 47% use it “in part or totally for medicinal purposes,” and 10% use it solely for medicinal purposes (Ko et al., 2016). Research shows that in 2004, about 4% of Canadians over the age of 14 reported using Cannabis in the past year for self-identified medical problems (Schauer et al., 2016). Cannabis remains the most commonly used drug globally, with more than 4% of the global population aged 15–64 (approximately 209 million people) using Cannabis in 2020, a 23% increase from 170 million in 2010 (Richards et al., 2020). Approximately 27% of Israeli adults consumed Cannabis in 2020, making it the country with the highest incidence of Cannabis use as of that year. (Bar-Or et al., 2021). Comparatively, the United States has a lower incidence of Cannabis use, with approximately 17% of the adult population reported to have consumed Cannabis within the same period (Sarvet et al., 2018). In Europe, Czechia has the highest incidence of Cannabis use of 11.1% among their adult population (Arnarsson et al., 2018). Forecasts put the global Cannabis market at $82.3 billion in 2027, a significant projection of 24.3% with $27.7 billion recorded in 2022 (Chen et al., 2021). The United Nations Office on Drugs and Crime (UNODC) identifies Morocco as the largest producer of ‘psychoactive marijuana plants’ worldwide (Kitchen et al., 2022). However, in terms of revenue generation, the United States leads in terms of the sale of medical Cannabis, with an annual total of 10 billion dollars, a significant portion of which comes from therapeutic marijuana (Kilmer and MacCoun, 2017). In Europe, Germany leads in the sale of medical marijuana, with an estimate 87.2 million dollars (Häuser et al., 2018). Between 1995 and 2005, 19 African countries reported the cultivation of Cannabis within their borders. In 2005, worldwide, Cannabis production was estimated at 42,000 metric tons, with Africa alone accounting for 25% of the total (Akyeampong, 2005).
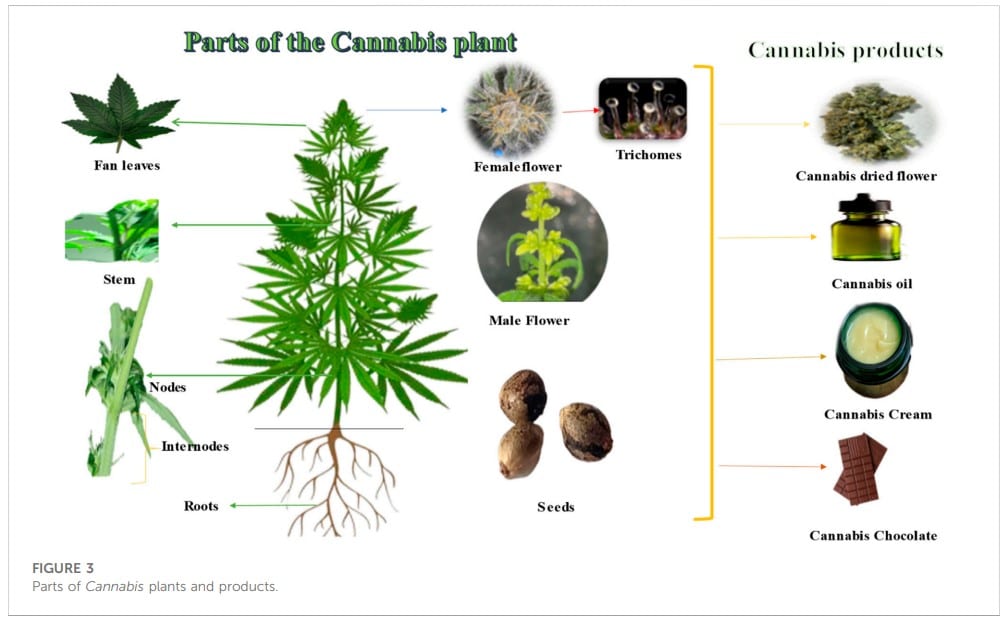
2.5 Morphological characteristics of Cannabis
Cannabis sativa L. is an annual plant that can reach up to 5 m in height and has upright stems with palmate leaves consisting of 5–7 linear-lanceolate leaflets (Figure 3). Male flowers lack petals and grow in axillary or terminal panicles, while female flowers have a single ovule and a perianth that is tightly attached (Farag and Kayser, 2017; Bonini et al., 2018). Trichomes, which are glandular protuberances that cover the plant’s leaves, bracts, and stems, are present in high concentrations (Bonini et al., 2018).
The fruit of each flower is a single small smooth light brownish-grey fruit that is then passed on to the next-generation. Female flowers grow at the end of the stem and in the axils. They have one ovule and a perianth that is tightly connected. Male flowers, on the other hand, have five yellowish petals and five anthers (Farag and Kayser, 2017).
3 Phytochemistry of Cannabis
The number of natural chemicals isolated from Cannabis sativa L. has not significantly increased in recent years, despite over 500 compounds being discovered so far (Pellati et al., 2018; Al Ubeed et al., 2022). In 1980, 423 compounds were discovered, which grew to 483 by 1995 (Matsuda et al., 1990; Dos Santos and Romão, 2023). Currently, 566 compounds have been identified and isolated which constitutes over 18 classes of different secondary metabolites found in the plant. These substances have been found to be highly abundant in the flowers and leaves of the plant (Kopustinskiene et al., 2022; Odieka et al., 2022). Out of this number, 125 are cannabinoids, 198 are non-cannabinoids and 120 are terpenes,
constituting a total of 443. The rest of the substances identified in the plant in 2021 include 2 alkaloids, 34 flavonoids, 42 phenols and 3 sterols (Al Ubeed et al., 2022). The aromatic quality of female Cannabis plants is due to the terpenes they produce, such as pinene, limonene, terpineol, and borneol (McPartland et al., 2001). These terpenes have insect-repellent properties and inhibit the growth of neighboring vegetation. The glandular trichomes on the plant produce a resin that acts as a sophisticated defense mechanism against insects and has the potential to serve as an antibiotic and antifungal agent. These trichomes contain secondary metabolites like phytocannabinoids and terpenoids that are responsible for the plant’s defense and interaction with herbivores and pests, as well as its characteristic scent (Andre et al., 2016). The various phytochemicals are summarized below.
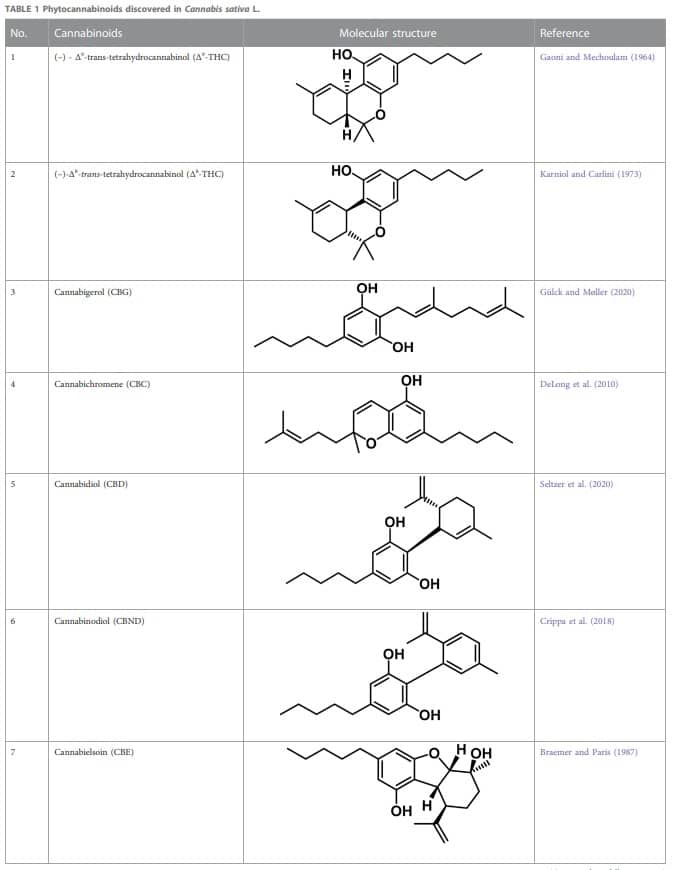
3.1 Cannabinoids
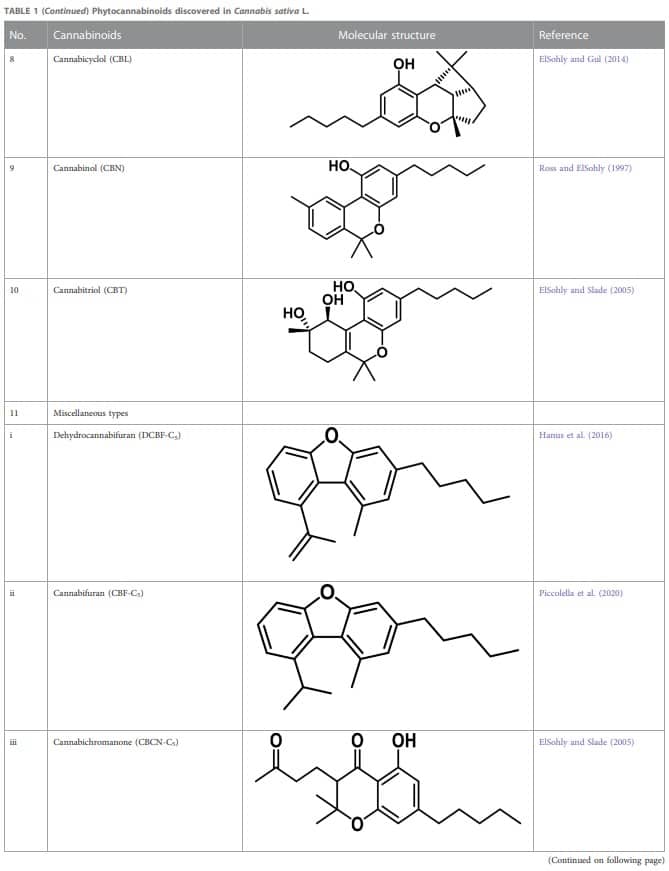
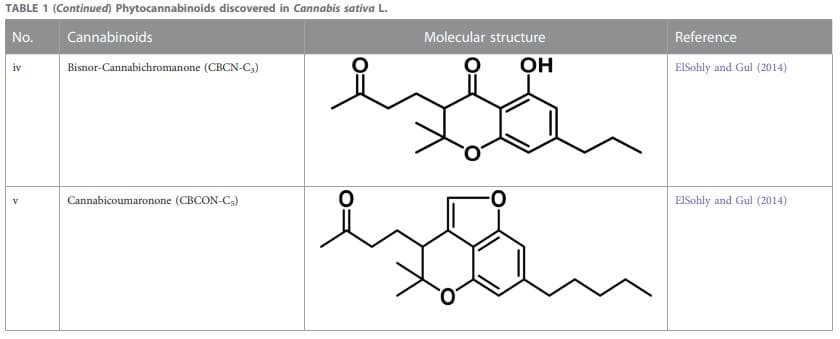
Therapeutic marijuana has a high level of tetrahydrocannabinol (THC), but minimal levels of cannabidiolic acid (CBDA) and cannabidiol (CBD). Cannabinoids undergo decarboxylation during drying, storage, and thermal processing, converting from an acidic to a neutral state. There are now many types of cannabinoids, not just those found in Cannabis, and the term “phytocannabinoids” has been used for those that naturally come from the plant (Radwan et al., 2021). A total of 120 phytocannabinoids have been identified and divided into 11 categories (Berman et al., 2018; Bonn-Miller et al., 2018). Table 1 lists the 11 subclasses of 120 phytocannabinoids.
3.1.1 (−)-Delta-9-trans-tetrahydrocannabinol (Δ9– THC) type
Gaoni and Mechoulam (1971) discovered the structure of Δ9– THC and explained its psychoactive properties. Rhee et al. (1997) used X-ray and proton magnetic resonance (1H NMR) studies to determine the precise conformation of Δ9-THC (Rhee et al., 1997). Dewey (1986) identified Δ9-THCA-A from Cannabis extract, which is photosensitive and cannot form crystals (structure as compound 2 shown in Table 1) (Dewey, 1986). Devane et al. (1988) discovered Δ9-THCA-B (compound 3 in Table 1) from Cannabis. Cannabis sole, a flat form of illicit Cannabis, was eluted from the silicic acid matrix using a 1:1 diethyl ether/petroleum ether solution. Δ9-THCA-B was shown to be more polar than Δ9– THCA-A in thin-layer chromatography (TLC). The determination of the crystalline structure of Δ9-THCA-B was due to the differences in biochemical properties between Δ9– THCA-B and Δ9-THCA-A (Galal et al., 2009).
Romano and Hazekamp (2019) isolated Δ9– tetrahydrocannabivarin (Δ9-THCV) using a mixture of 5 g of Cannabis and 200 mL of petroleum ether and dissolved it in 100 mL of absolute ethyl alcohol (EtOH) (Romano and Hazekamp, 2019). Spectroscopic evidence for Δ9-trans tetrahydrocannabidiolic acid (Δ9-THCVA) was reported by Matsuda et al. (1990), followed by mass spectrometric evidence data (Pate, 1994). The analysis of 51 samples sourced from various geographic regions led to research on the C3 homologs of Cannabis (Turner et al., 1973). Balcke et al. (2014) discovered a new homologue of Δ9-THC with a methyl side chain, 9- tetrahydrocannabiorcol (Δ9-THC-C1), in an extract of Brazilian Cannabis (Balcke et al., 2014). The concentration of Δ9-THC
C1 was low, so it was not expected to have a significant impact on the drug’s biological action. Dewey (1986) identified Δ9-trans-THCA C4 and Δ9-trans-THC-C4 using GC-MS, as well as Δ9-trans tetrahydrocannabiorcolic acid (Δ9-THCA-C1) (Balcke et al., 2014). Several techniques, including NMR spectroscopy and Gas Chromatography-Mass Spectrometry (GC-MS), were used to identify monoterpene or sesquiterpene esters of 9- tetrahydrocannabinolic acid A in Cannabis sativa L. These esters were found to be precursors to Δ9-THC and were broken down into their constituents when subjected to high temperatures during GC-MS analysis (Caspi et al., 2005). Chromatographic methods, such as vacuum liquid chromatography (VLC), High-performance liquid chromatography (HPLC), and Supercritical fluid chromatography (SPC) were used to isolate these cannabinoid esters from high-potency C. sativa varieties. Cannabisol, a dimeric cannabinoid, was also isolated using flash silica gel column chromatography from Cannabis samples that contained a significant amount of CBG (Costa et al., 2007). Eight new substances of the tetrahydrocannabinol family are listed in Table 2.
3.1.2 Cannabigerol (CBG) type
Cannabigerol (CBG) is the first substance purified from Cannabis resin (CBG-C5, compound 5 in Table 1) (Mechoulam and Shvo, 1963). Mechoulam et al. (1995) were the first to describe the condensation of geranyl pyrophosphate in the formation of CBG. Mechoulam et al. (1995) discovered that cannabidiolic acid (CBGA) was the most polar acid component. They also found the methyl ester of CBGA in the acidic part of a single extract of Cannabis (Mechoulam et al., 1995).
Cannabigerovarinic acid (CBGVA–the structure of compound 1 in Table 3) isolated from an extract of the dried leaves of Thai Cannabis was found to be a minor component of the extract (Thomas, 1996; Van Os et al., 2002). After extraction of the acid fraction from the leaves using silica gel column chromatography, the acid fraction was eluted from the dried leaves using a mixture of hexane, ethyl
acetate, and a ratio of 5:1 of benzene to acetone. The transparent needle-like CBGVA crystals were obtained following recrystallization in hexane: ethyl acetate solution in a ratio of 3:1. Cannabinerolic acid (CBRA) and cannabigerolic acid (CBGA) are both acidic cannabinoids that are produced in the Cannabis plant. The primary difference between the two is the location of the double bond in their molecular structures (Taura et al., 1996). CBGA is the precursor to many of the other cannabinoids found in Cannabis, including THC and CBD. It is synthesized by the plant from olivetolic acid and geranyl pyrophosphate. CBGA can be further converted into THCA, CBDA, or CBCA, which are then decarboxylated to produce THC, CBD, or CBC (cannabichromene) (Morimoto et al., 1998). Taura et al. (1995) described a procedure to purify cannabinerolic acid from an air-dried Mexican strain of C. sativa by extracting the leaves with benzene.
The extraction was concentrated and loaded onto a silica gel column, then extracted with a 9:1 (v/v) benzene/acetone mixture after dissolving the residue in acetone and removing any insoluble particulates. High-potency cannabigerolic acid esters, i.e., γ-eudesmyl cannabigerolate and α-cadinyl cannabigerolate were also recovered from C. sativa in another study (Kinghorn et al., 2017). The hexane extract of Cannabis was purified by chromatography to obtain the two cannabigerolic acid esters. Both γ-eudesmyl cannabigerolate and α-cadinyl cannabigerolate were shown to be esters of CBGA by the data obtained from their respective spectroscopic analyses (Wallace et al., 2001). van Winkel (2011) identified six substances using flash silica gel analysis of a hexane extract, including 5- acetyl-4-hydroxycannabigerol, 4-acetoxy-2-geranyl-5-hydroxy 3-n-pentylphenol, (±)-6,7-trans-epoxycannabigerolic acid, (±)-6,7-cis-epoxycannabigerolic acid and (±)-6,7-cis epoxycannabigerol (Van Waes et al., 2012). Appendino et al. (2008) isolated a novel, polar dihydroxy cannabigerol derivative (carmagerol) from the Cannabis leaves. Taylor et al. (2010) identified sesquicannabigerol, a lipophilic analogue of cannabigerol, in the waxy section of the fiber hemp cultivar Carma. Methanolic potassium hydroxide (·KOH) was used to hydrolyze the wax, and it was purified using gravity silica gel
